Gut Health Advancements: Pediatric Fecal Microbiota Transplantation Guidance Released by AAP
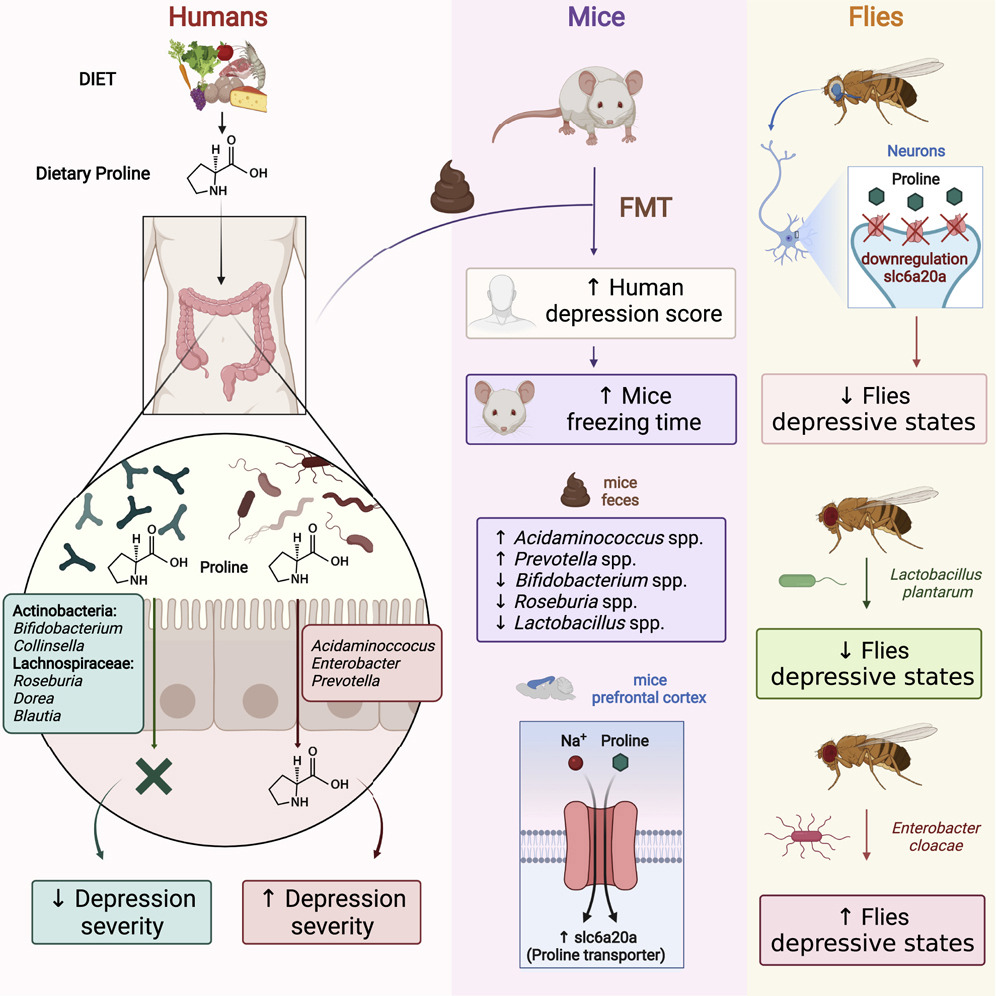
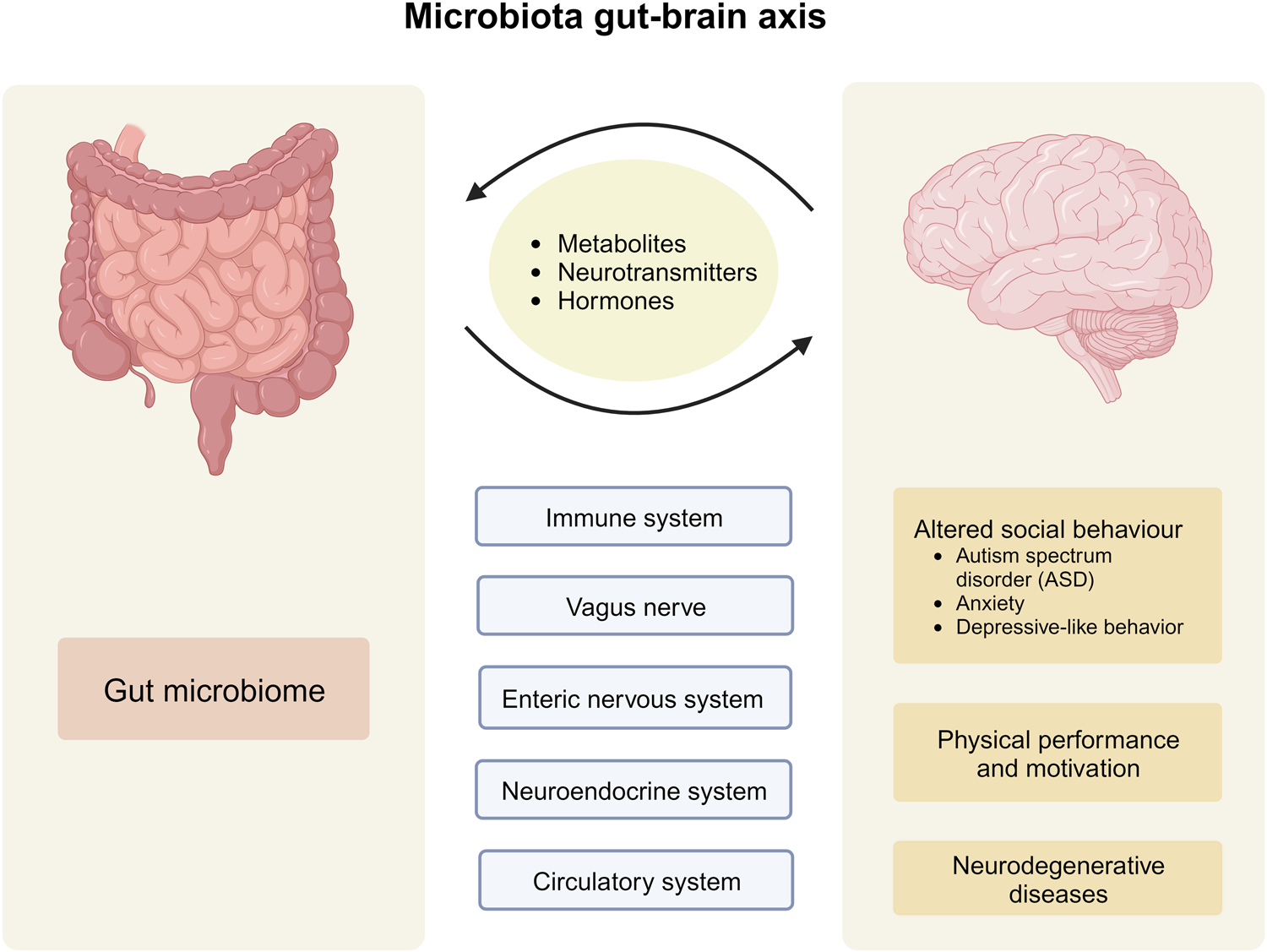
A clinical report in "Pediatrics", released by the Amercian Academy of Pediatrics (AAP), recommends fecal microbiota transplantation (FMT) for children with moderate-to-severe or recurrent Clostridioides difficile infection (CDI).
Key highlights of the study:
- This recommendation is based on the current evidence supporting the effectiveness of FMT in treating CDI in adults.
- Authors, including Maria Oliva-Hemker, M.D., from Johns Hopkins University, highlight the lack of prospective clinical trials in children for FMT, but support its use in pediatric CDI cases.
- FMT is currently not advised for treating other medical conditions in children.
- For safety, home-based, do-it-yourself FMT is discouraged; it should be performed in experienced centers.
- There are no regulatory standards for fecal preparations used in FMT, and the long-term effects of FMT are still unknown.
- The field of antimicrobial therapies is rapidly advancing, with expectations of commercial products for CDI treatment in the future.
- The authors stress the importance of including children in clinical trials for these upcoming microbial therapeutic products.
- Published by Maria Oliva-Hemker et al, the report in "Pediatrics" serves as a guide for pediatricians and families considering FMT for CDI treatment.
More information:
Journal Reference: Pediatrics, DOI: 10.1542/peds.2023-062922.
Image credits: Freepik.