Alerts
Critical Evaluation of Faecal Microbiome Preservation using Metagenomic Analysis
 The Organizing Committee of Targeting Microbiota 2021 Congress is honored to announce the particpation of Dr. Alena Pribyl from Microba, USA.
The Organizing Committee of Targeting Microbiota 2021 Congress is honored to announce the particpation of Dr. Alena Pribyl from Microba, USA.
Dr. Pribyl will present their recent investigation on "Critical Evaluation of Faecal Microbiome Preservation using Metagenomic Analysis".
Additionally, she recently published a related article in Nature about their study.
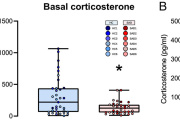
Dr. Pribyl demonstrates that accurate preservation of faecal samples is critical as more studies seek to use the gut microbiome to identify biomarkers of disease. In her research, she undertakes an in-depth evaluation of faecal preservation methods using shotgun metagenomics to identify the best methods for use at room temperature. These methods included RNALater, OMNIGene-GUT, a dry BBL swab, LifeGuard, and a novel method for preserving faecal samples, a Copan FLOQSwab in an active drying tube (FLOQSwab-ADT). Along with her team, she further evaluated the best performing method, the FLOQSwab-ADT, at various temperatures to determine its range of use and found it maintained its performance across all temperatures.
Targeting Microbiota 2021 Congress
October 20-22, 2021 - Paris, France & Online
www.microbiota-site.com
Honeybees Microbiome: Recent Advances and Perspective on Compartmentalization in the Adult Gut

Prof. Daniele Daffonchio, from King Abdullah University, Saudi Arabia, will join us at the 8th World Congress on Targeting Microbiota.
Prof. Daffonchio will talk about "Honeybees Microbiome: Recent Advances and Perspective on Compartmentalization in the Adult Gut", mainly focuing on the microbial components of forager honeybees and their compartmentalization along the gut protions. Prof. Daffonchio will explain the results of his studies and the observed changes in the distribution and abundance of microbial components in the gut.
Targeting Microbiota 2021 Congress
October 20-22, 2021 - Paris, France & Online
www.microbiota-site.com
Microbial Endocrinology as a Framework for understanding the Avian Microbiome in a Post-Antibiotic World
 Dr. Joshua Lyte from USDA Agricultural Research Service, USA will join the Targeting Microbiota 2021 Congress and will give a presentation entitled "Microbial Endocrinology as a Framework for Understanding the Avian Microbiome in a Post-Antibiotic World".
Dr. Joshua Lyte from USDA Agricultural Research Service, USA will join the Targeting Microbiota 2021 Congress and will give a presentation entitled "Microbial Endocrinology as a Framework for Understanding the Avian Microbiome in a Post-Antibiotic World".
In his talk, Dr. Lyte will highlight about: The poultry industry has supplemented feed with antibiotics for decades in order to improve avian growth performance, maintain intestinal health, and suppress colonization by foodborne pathogens that cause severe diseases in human consumers. With this decades-old practice of antibiotic feed supplementation rapidly coming to an end, the poultry industry is examining how the microbiome can be harnessed to promote intestinal health and prevent colonization by foodborne pathogens. The evolutionary, neurochemical-based, cross-talk between the microbiome and the host, known as microbial endocrinology, provides a framework to elucidate the mechanisms by which the microbiome influences poultry health and exclusion of foodborne pathogens. As members of the microbiota both produce and recognize the same neurochemicals that are produced by the host’s intestinal nervous system and enteroendocrine cells, use of a microbial endocrinology-based context represents a unique approach to identify relevant mechanisms as well as design interventions to promote poultry health, and ultimately human health as well.
Targeting Microbiota 2021 Congress
October 20-22, 2021 - Paris, France & Online
www.microbiota-site.com
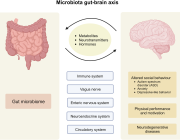
Microbiota regulate social behavior via stress response neurons in the brain
 Dr. Wei-Li Wu from California Institute of Technology, USA will join the Targeting Microbiota 2021 Congress and give a presentation entitled "Microbiota regulate social behavior via stress response neurons in the brain".
Dr. Wei-Li Wu from California Institute of Technology, USA will join the Targeting Microbiota 2021 Congress and give a presentation entitled "Microbiota regulate social behavior via stress response neurons in the brain".
This talk will be about: The microbiome modulates neuronal activity in specific brain regions of mice to regulate canonical stress responses and social behaviours. Specific gut bacteria can restrain the activation of the hypothalamus-pituitary-adrenal axis. The microbiome can affect social behaviours through discrete neuronal circuits that mediate stress responses in the brain.
Targeting Microbiota 2021 Congress
October 20-22, 2021 - Paris, France & Online
www.microbiota-site.com
Fecal Microbiota and Metabolite Profiles in IBD and IBS
 Prof. Lena Öhman from University of Gothenburg, Sweden will join the Targeting Microbiota 2021 Congress and will give a presentation entitled "Fecal Microbiota and Metabolite Profiles in IBD and IBS".
Prof. Lena Öhman from University of Gothenburg, Sweden will join the Targeting Microbiota 2021 Congress and will give a presentation entitled "Fecal Microbiota and Metabolite Profiles in IBD and IBS".
Prof. Öhman's research group is successfully focusing on describing gut microbiota, immune profile and the link to disease profile, as well as therapy outcome in patients with inflammatory bowel disease (IBD) and patients with with irritable bowel syndrome (IBS).
Patients with inflammatory bowel disease suffer from a chronic inflammation of the intestinal tract. The aetiology of the intestinal inflammation is still unknown but is thought to arise due to a dysregulated immune response to gut microbiota. The disease cannot be cured, although many IBD patients benefit from antibody therapy, but the effect is highly variable and at least 30% of the patients undergoing this treatment experience no clinical improvement. Today, it is not possible to predict the patients’ disease course or response to biological therapy. Hence, there is an urgent need for biomarkers predicting clinical disease course and therapeutic outcome in patients with IBD.
Irritable bowel syndrome (IBS) is considered to be one of the most frequent clinical problems in gastroenterology with an estimated prevalence in the community between 10 to 25 %. Despite the high frequency, effective therapeutic strategies for IBS are limited with average therapeutic gains over placebo and there is a clear unmet medical need for treating IBS.
We hypothesize that gut microbiota and the immune system influence disease course and therapeutic outcome in the patients with IBD and IBS, respectively. The aim of this project is therefore to establish how gut microbiota and immune activity influence disease course and identify microbiotic and immunological indicators of therapy outcome.
Targeting Microbiota 2021 Congress
October 20-22, 2021 - Paris, France & Online
www.microbiota-site.com
The Promise of Microbiota Modulation during COVID-19 Pandemic
 Prof. Siew C Ng from The Chinese University of Hong Kong will present her recent study entitled "The Promise of Microbiota Modulation during COVID-19 Pandemic".
Prof. Siew C Ng from The Chinese University of Hong Kong will present her recent study entitled "The Promise of Microbiota Modulation during COVID-19 Pandemic".
During her presentation, Prof. Ng will present new data on impact of gut microbiota on post acute covid syndrome and the role of targeted microbiota modulation during SARS Cov2 Pandemic, and its relationship with COVID-19 vaccination.
Targeting Microbiota 2021 Congress
October 20-22, 2021 - Paris, France & Online
www.microbiota-site.com
Nonlinear Machine Learning Pattern Recognition and Bacteria-Metabolite Multilayer Network Analysis of Perturbed Gastric Microbiome
 Dr. Carlo V. Cannistraci from Tsinghua University, China will give a presentation entitled "Nonlinear Machine Learning Pattern Recognition and Bacteria-Metabolite Multilayer Network Analysis of Perturbed Gastric Microbiome".
Dr. Carlo V. Cannistraci from Tsinghua University, China will give a presentation entitled "Nonlinear Machine Learning Pattern Recognition and Bacteria-Metabolite Multilayer Network Analysis of Perturbed Gastric Microbiome".
During his presentation, Dr. Carlo V. nistracCani will state that the stomach is inhabited by diverse microbial communities, co-existing in a dynamic balance. Additionally, long-term use of drugs such as proton pump inhibitors (PPIs), or bacterial infection such as Helicobacter pylori, cause significant microbial alterations. Yet, studies revealing how the commensal bacteria re-organize, due to these perturbations of the gastric environment, are in early phase and rely principally on linear techniques for multivariate analysis. In this talk, he discloses the importance of complementing linear dimensionality reduction techniques with nonlinear ones to unveil hidden patterns that remain unseen by linear embedding. Then, he proves the advantages to complete multivariate pattern analysis with differential network analysis, to reveal mechanisms of bacterial network re-organizations which emerge from perturbations induced by a medical treatment (PPIs) or an infectious state (H. pylori). Finally, he shows how to build bacteria-metabolite multilayer networks that can deepen our understanding of the metabolite pathways significantly associated to the perturbed microbial communities.
Targeting Microbiota 2021 Congress
October 20-22, 2021 - Paris, France & Online
www.microbiota-site.com
The Horse Gut Microbiome Responds in a Highly Individualized Manner to Forage Lignification
 Dr. Andres Gomez from University of Minnesota-Twin Cities, USA will join the Targeting Animal Microbiota Symposium and give a presentation entitled "The Horse Gut Microbiome Responds in a Highly Individualized Manner to Forage Lignification".
Dr. Andres Gomez from University of Minnesota-Twin Cities, USA will join the Targeting Animal Microbiota Symposium and give a presentation entitled "The Horse Gut Microbiome Responds in a Highly Individualized Manner to Forage Lignification".
In this talk, Dr. Gomez will show how different horses harbor unique fecal microbiomes, which are modulated in a horse-specific manner upon dietary changes. Specifically, that when the percent of lignin is changed in forage, the equine gut microbiome is not modulated in the same way across different individuals. Instead, each horse microbiome reacts differently to the same dietary change.
Targeting Microbiota 2021 Congress
October 20-22, 2021 - Paris, France & Online
www.microbiota-site.com
Role of microbiome in cardiometabolic health
 Dr. Noel T. Mueller from Johns Hopkins University Bloomberg School of Public Health, USA will join the Targeting Microbiota 2021 congress and will present his recent study on Role of microbiome in cardiometabolic health.
Dr. Noel T. Mueller from Johns Hopkins University Bloomberg School of Public Health, USA will join the Targeting Microbiota 2021 congress and will present his recent study on Role of microbiome in cardiometabolic health.
Dr. Mueller will also highlight the Effects of metformin on microbiome and metabolites.
Targeting Microbiota 2021 Congress
October 20-22, 2021 - Paris, France & Online
www.microbiota-site.com
Real-time monitoring of ruminal microbiota reveals their roles in dairy goats during subacute ruminal acidosis
 Dr. Shengru Wu, Associate professor of Northwest A&F University, China will join the Targeting Microbiota 2021 Congress which will be held on October 20-22, 2021 and give a presentation entitled "Real-time monitoring of ruminal microbiota reveals their roles in dairy goats during subacute ruminal acidosis".
Dr. Shengru Wu, Associate professor of Northwest A&F University, China will join the Targeting Microbiota 2021 Congress which will be held on October 20-22, 2021 and give a presentation entitled "Real-time monitoring of ruminal microbiota reveals their roles in dairy goats during subacute ruminal acidosis".
Summary of Talk: Ruminal microbiota changes frequently with high grain diets and the occurrence of subacute ruminal acidosis (SARA). Significant bacterial differences between goats from the SARA and healthy groups are identified at every hour for six continuous hours after feeding, and 29 common differential genera between two groups over 6 h after feeding are all related to the altered pH and lipopolysaccharides. Transplanting the microbiota from donor goats with SARA could induce colonic inflammation in antibiotic-pretreated mice. Overall, significant differences in the bacterial community and rumen fermentation pattern between the healthy and SARA dairy goats are real-time monitored, and then tested using ruminal microbe transplantation to antibiotic-treated mice.
Targeting Microbiota 2021 Congress
October 20-22, 2021 - Paris, France & Online
www.microbiota-site.com
The Eubiotic Potential Of Tannins In Piglets' Nutrition

Dr. Jakub Piwowarski from Medical University of Warsaw, Poland. Dr. Piwowarski will present his recent study entitled The Eubiotic Potential Of Tannins In Piglets' Nutrition.
Tannins are commonly considered as anti-nutritional factors in piglets' nutrition. On the other hand, certain tannin-containing plants are well known for their anti-diarrheal properties, which have been utilized since ancient times in human and veterinary medicine but had been superseded by antibiotics, since discovery of penicillin in 1928 and introduction of antimicrobials in farm animal production since the 1950s. Enterotoxigenic Escherichia coli (ETEC)- induced diarrhoea in piglets is one of the most important health conditions in pigs farming. Decades of extensive antibiotics use in prevention and therapy of infections in animals significantly contributed to the spread of antimicrobial resistance, leading to the restrictions on their use in farm animals. As the consequence, the development of novel preventive and therapeutic strategies targeted on maintaining piglets gut health, which are based on pleiotropic mechanisms, is urgently needed.
The conducted studies have shown that selected tannin-rich plant formulations are able to significantly inhibit the ETEC growth and its adhesion to intestinal epithelial cell monolayers. The relevant stimulation of IPEC-J2 epithelial cells monolayers formation through enhancement of tight junction proteins production was also observed. Despite the determined anti-ETEC properties, the tested tannin sources did not negatively affect alpha diversity and metabolism of intestinal microbiota of post-weaning piglets ex vivo. Certain changes in microbial taxa abundances were induced, some of which correlated with the formation of postbiotic metabolites, namely urolithins, which proven anti-inflammatory properties can be beneficially contribute to gut health of piglets during the weaning period.
The conducted studies support the historically attributed anti-diarrheal properties of tannin-containing plant preparations revealing their eubiotic effects, that not only respect the ecological context of preserving the homeostasis of intestinal microbiota but also support the intestinal epithelium development in post-weaning piglets. The obtained results serve as an initial point for further studies on development of novel, sustainable feed additives dedicated to farm animals being scientifically based alternatives to antibiotics.
Targeting Microbiota 2021 Congress
October 20-22, 2021 - Paris, France & Online
www.microbiota-site.com
Linking Lactic Acid Bacteria from Food with the Gut Microbiome
 Dr. Edoardo Pasolli, from University of Naples Federico II, Department of Agricultural Sciences, Italy, will join the Targeting Microbiota Congress which will be held on October 20-22, 2021 and will present a talk entitled "Linking Lactic Acid Bacteria from Food with the Gut Microbiome".
Dr. Edoardo Pasolli, from University of Naples Federico II, Department of Agricultural Sciences, Italy, will join the Targeting Microbiota Congress which will be held on October 20-22, 2021 and will present a talk entitled "Linking Lactic Acid Bacteria from Food with the Gut Microbiome".
According to his latest researches, he confirms that large quantities of live lactic acid bacteria are consumed within fermented foods, but it is not yet known to what extent the LAB they ingest become members of the gut microbiome. By analysis of almost ten thousand metagenomes from human samples, Dr. Pasolli shows that the prevalence and abundance of LAB species in stool samples is generally low and linked to age, lifestyle, and geography. Moreover, we demonstrate that closely related LAB strains occur in both food and gut environments and provides unprecedented evidence that fermented foods can be indeed regarded as a possible source of LAB for the gut microbiome.
Targeting Microbiota 2021 Congress
October 20-22, 2021 - Paris, France & Online
www.microbiota-site.com
Fecal Microbiota Transplantation in Dogs and Cats

The Scientific committee is honored to announce the participation of Dr. Frederic Gaschen, from Louisiana State University School of Veterinary Medicine, USA. Dr. Gaschen will present his recent study entitled Fecal Microbiota Transplantation in Dogs during the symposium.
Abstract: In people, fecal microbiota transplantation is recognized as the best treatment modality for recurrent Clostridioides difficile infection in people, and its value is currently investigated in the treatment of other diseases associated with an abnormal gut microbiome. In dogs, intestinal dysbiosis has been documented in many acute and chronic digestive diseases as well as in diseases of other organ systems. There are only few published studies evaluating the benefits of fecal microbiota transplantation (FMT) in canine gastrointestinal disorders. They provide evidence that FMT may be beneficial in the treatment of acute intestinal diseases and hope that the technique might also be useful for the management of chronic enteropathies.
More about the article: https://doi.org/10.1016/j.cvsm.2020.09.012
Targeting Microbiota 2021 Congress
October 20-22, 2021 - Paris, France & Online
www.microbiota-site.com
Recent Advances on the Fish Skin Microbiomes – Challenges in deciphering their shaping factors
 Dr. Amir Szitenberg from Arava and Dead Sea Science Center, Israel will join the Targeting Microbiota Congress which will be held on October 20-22, 2021 and will present a talk entitled "Recent advances on the fish skin microbiomes – challenges in deciphering their shaping factors".
Dr. Amir Szitenberg from Arava and Dead Sea Science Center, Israel will join the Targeting Microbiota Congress which will be held on October 20-22, 2021 and will present a talk entitled "Recent advances on the fish skin microbiomes – challenges in deciphering their shaping factors".
Summary of Talk: The gut, skin and gill microbiota in fish are of paramount importance to their nutrition, health, immune system, interactions with the changing environment and their general well being. On top of co-phylogenetic influences, these microbiota are affected by the geography, the local physicochemistry and ecology, as well as the fish species life-style. Disentangling the most important factors in the wild is challenging, and yet it is important in order to understand the interface of aquaculture and conservation, or to develop effective and viable biotechnological processes. This talk will discuss recent advances in our understanding of these factors and how this knowledge can be harnessed to grow and conserve fish species.
Targeting Microbiota 2021 Congress
October 20-22, 2021 - Paris, France & Online
www.microbiota-site.com
Session Dedicated to Diet as Microbiome and Epigenetic Modulator in Diabetes and Obesity
 Dr. Annalisa Terranegra from Sidra Medical and Research Center, Qatar, will chair a session dedicated to Diet as Microbiome and Epigenetic Modulator in Diabetes and Obesity during the congress.
Dr. Annalisa Terranegra from Sidra Medical and Research Center, Qatar, will chair a session dedicated to Diet as Microbiome and Epigenetic Modulator in Diabetes and Obesity during the congress.
The main topic of this session is to discuss epigenetic activity of the microbiota and its metabolites as mechanism controlling the host-microbe interaction.
If you wish to present a short oral presentation during this session, please submit your abstract here.
Among the speakers of this session:
 Gut Microbiota as Important Mediator Between Diet and DNA Methylation and Histone Modifications in the Host Gut Microbiota as Important Mediator Between Diet and DNA Methylation and Histone Modifications in the HostDina Bellizzi, University of Calabria, Italy |
Targeting Microbiota 2021 Congress
October 20-22, 2021 - Paris, France & Online
www.microbiota-site.com
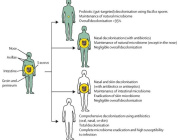
A session dedicated to Microbiota and Antibiotics
During the Targeting Microbiota 2021 Congress, a special session dedicated to "Microbiota and Antibiotics" will be organized and will be chaired by Dr. Maria Cecilia Giron, University of Padova, Italy.
Among the Speakers:
|
Summary: Antibiotics are life-saving medications but any time they are administered, they can cause side effects and contribute to the development of antibiotic resistance. Disruptions in the neuroimmune interactions between gut microbiota and enteric nervous system during critical developmental windows by antibiotic exposure, have been linked to increased susceptibility of the host to several diseases mediated by the microbiota-gut-brain axis, ranging from irritable bowel syndrome to psychiatric and neurologic disorders.
|
|
Summary: The gastrointestinal (GI) tract is a complex multifunctional organ where commensals microbes and their related metabolites in the lumen modulate several neuronal, immune, and endocrine cellular systems in the gut wall, such as the enteric nervous system or outside the GI tract, such as the brain. The mechanisms behind this intimate crosstalk are beginning to be elucidated, and they are crucial not only for understanding the pathophysiology of functional intestinal diseases but also for identifying biomarkers that describe and help cure neurological disorders with GI comorbidities. This talk will explore the recent research on antibiotics as a tool to explore the gut microbiota-host neuro-endocrine-immune interactions and the relevance of these studies for the disorders of the microbiota-gut-brain axis. |

Joshua Lyte, University of Arkansas, USA Summary: For decades the poultry industry has supplemented feed with antibiotics in order to improve avian growth performance, maintain intestinal health, and suppress colonization by foodborne pathogens that cause severe diseases in human consumers. With this decades-old practice of antibiotic feed supplementation rapidly coming to an end, the poultry industry is examining how the microbiome can be harnessed to promote intestinal health and prevent colonization by foodborne pathogens. The evolutionary, neurochemical-based, cross-talk between the microbiome and the host, known as microbial endocrinology, provides a framework to elucidate the mechanisms by which the microbiome influences poultry health and exclusion of foodborne pathogens. As members of the microbiota both produce and recognize the same neurochemicals that are produced by the host’s intestinal nervous system and enteroendocrine cells, use of a microbial endocrinology-based context represents a unique approach to identify relevant mechanisms as well as design interventions to promote poultry health, and ultimately human health as well. |
If you wish to present a short oral presentation during this session, please submit your abstract here.
Targeting Microbiota 2021 Congress
October 20-22, 2021 - Paris, France & Online
www.microbiota-site.com
The Microbiome as a Bridge Linking Antibiotics and Intestinal Function
 Dr. Valentina Caputi, from the University of Arkansas, USA, will join the Targeting Microbiota 2021 Congress which will be held on October 20-22, 2021 at UNESCO Paris & Online.
Dr. Valentina Caputi, from the University of Arkansas, USA, will join the Targeting Microbiota 2021 Congress which will be held on October 20-22, 2021 at UNESCO Paris & Online.
During the conference, Dr. Caputi will present "The Microbiome as a Bridge Linking Antibiotics and Intestinal Function" in session which will be shared with Dr. Maria Cecilia and Dr. Joshua Lyte.
Summary of Talk: The gastrointestinal (GI) tract is a complex multifunctional organ where commensals microbes and their related metabolites in the lumen modulate several neuronal, immune, and endocrine cellular systems in the gut wall, such as the enteric nervous system or outside the GI tract, such as the brain. The mechanisms behind this intimate crosstalk are beginning to be elucidated, and they are crucial not only for understanding the pathophysiology of functional intestinal diseases but also for identifying biomarkers that describe and help cure neurological disorders with GI comorbidities. This talk will explore the recent research on antibiotics as a tool to explore the gut microbiota-host neuro-endocrine-immune interactions and the relevance of these studies for the disorders of the microbiota-gut-brain axis.
Targeting Microbiota 2021 Congress
October 20-22, 2021 - Paris, France & Online
www.microbiota-site.com
Gut Microbiota Interactions with Cognitive Function

Prof. José-Manuel Fernández-Real from Hospital de Girona "Dr Josep Trueta", Universitat de Girona, Spain will join the Targeting Microbiota 2021 Congress which will be held on October 20-22, 2021 and give a presentation entitled "Gut Microbiota Interactions with Cognitive Function".
Summary of Talk:
- Gut microbiome composition and functionality have been recently found to be linked to immediate and short-term memory and several tests evaluating inhibitory control in subjects with and without obesity.
- Brain structures associated with these cognitive domains were also associated with gut microbiome alterations.
- The impairments in immediate memory and in inhibitory control of the donors were phenocopied to recipient mice through faecal microbiota transplantation, resulting in alterations of memory, reversal learning and changes in mouse brain transcriptomics.
Targeting Microbiota 2021 Congress
October 20-22, 2021 - Paris, France & Online
www.microbiota-site.com
Deciphering the Effect of Bacteria on Protein Conformational Diseases
 Dr. Daniel M Czyz from University of Florida, USA will join the Targeting Microbiota 2021 congress which will be held on October 20-22, 2021 and will present a presentation entitled "Deciphering the effect of bacteria on protein conformational diseases".
Dr. Daniel M Czyz from University of Florida, USA will join the Targeting Microbiota 2021 congress which will be held on October 20-22, 2021 and will present a presentation entitled "Deciphering the effect of bacteria on protein conformational diseases".
Summary of talk: Recent studies suggest that bacteria contribute to neurodegenerative protein conformational diseases. However, which bacteria and how do microbes contribute to these diseases is not well understood. Our work reveals which bacteria affect host proteostasis and potentially contribute to the pathogenesis of neurodegenerative diseases.
Targeting Microbiota 2021 Congress
October 20-22, 2021 - Paris, France & Online
www.microbiota-site.com
Gut-Brain Axis Regulation of Alzheimer’s Disease Pathology
 Prof. Giulio M Pasinetti from Icahn School of Medicine at Mount Sinai, USA will chair a session dedicated to Brain and Microbiota and will present the topic entitled "Gut-Brain Axis Regulation of Alzheimer’s Disease Pathology".
Prof. Giulio M Pasinetti from Icahn School of Medicine at Mount Sinai, USA will chair a session dedicated to Brain and Microbiota and will present the topic entitled "Gut-Brain Axis Regulation of Alzheimer’s Disease Pathology".
Targeting Microbiota 2021 Congress
October 20-22, 2021 - Paris, France & Online
www.microbiota-site.com
Evaluation of the Effect of Storage Methods on Fecal, Saliva, and Skin Microbiome Composition
 Dr. Se Jin Song from Center for Microbiome Innovation, Jacobs School of Engineering, US San Diego, USA will join the Targeing Microbiota 2021 Congress and will present her recent research article entitled "Evaluation of the Effect of Storage Methods on Fecal, Saliva, and Skin Microbiome Composition".
Dr. Se Jin Song from Center for Microbiome Innovation, Jacobs School of Engineering, US San Diego, USA will join the Targeing Microbiota 2021 Congress and will present her recent research article entitled "Evaluation of the Effect of Storage Methods on Fecal, Saliva, and Skin Microbiome Composition".
Article extract: As the number of human microbiome studies expand, it is increasingly important to identify cost-effective, practical preservatives that allow for room temperature sample storage. Here, we reanalyzed 16S rRNA gene amplicon sequencing data from a large sample storage study published in 2016 and performed shotgun metagenomic sequencing on remnant DNA from this experiment. Both results support the initial findings that 95% ethanol, a nontoxic, cost-effective preservative, is effective at preserving samples at room temperature for weeks. We expanded on this analysis by collecting a new set of fecal, saliva, and skin samples to determine the optimal ratio of 95% ethanol to sample. We identified optimal collection protocols for fecal samples (storing a fecal swab in 95% ethanol) and saliva samples (storing unstimulated saliva in 95% ethanol at a ratio of 1:2). Storing skin swabs in 95% ethanol reduced microbial biomass and disrupted community composition, highlighting the difficulties of low biomass sample preservation. The results from this study identify practical solutions for large-scale analyses of fecal and oral microbial communities.
Targeting Microbiota 2021 Congress
October 20-22, 2021 - Paris, France & Online
www.microbiota-site.com
More Articles...
- Human gut microbiome signature reflects healthy aging and predicts survival in the latest decades of life
- Gut Microbiota as Important Mediator Between Diet and DNA Methylation and Histone Modifications in the Host
- FMT 2021: Reorganization and Perspective
- The Microbiome of IBD and the Potential of Machine Learning



 Microbiota and Antibiotics: Introduction
Microbiota and Antibiotics: Introduction