Alerts
Human gut microbiome signature reflects healthy aging and predicts survival in the latest decades of life
 Dr. Sean M. Gibbons from Institute for Systems Biology, University of Washington, USA will join the Targeting Microbiota 2021 congress and will present his recent study on Human gut microbiome signature reflects healthy aging and predicts survival in the latest decades of life.
Dr. Sean M. Gibbons from Institute for Systems Biology, University of Washington, USA will join the Targeting Microbiota 2021 congress and will present his recent study on Human gut microbiome signature reflects healthy aging and predicts survival in the latest decades of life.
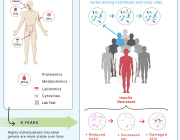
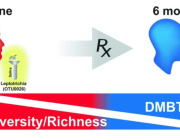
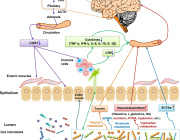
Prof. Gibbons highlights: The ecological dynamics of the human gut microbiome are characterized by rapid change in early life (0-3 years), followed by a long period of stability, and ending with steady changes associated with advanced age (> 65 years). While certain aging-related patterns in the gut microbiota have been linked to a concomitant decline in health, there is no clear definition of 'healthy aging' from the perspective of the gut microbiome. We leverage three independent, large, deeply phenotyped cohorts to show how increasing gut microbiome uniqueness and an associated rise in anti-inflammatory microbial metabolites in the blood are associated with healthier aging and increased survival in the latest decades of life.
Targeting Microbiota 2021 Congress
October 20-22, 2021 - Paris, France & Online
www.microbiota-site.com
Gut Microbiota as Important Mediator Between Diet and DNA Methylation and Histone Modifications in the Host
 Prof. Dina Bellizzi from University of Calabria, Italy will talk about "Gut Microbiota as Important Mediator Between Diet and DNA Methylation and Histone Modifications in the Host".
Prof. Dina Bellizzi from University of Calabria, Italy will talk about "Gut Microbiota as Important Mediator Between Diet and DNA Methylation and Histone Modifications in the Host".
The human gut microbiota is a complex ecosystem consisting of trillions of microorganisms that inhabit symbiotically on and in the human intestine. They carry out, through the production of a series of metabolites, many important metabolic functions that complement the activity of mammalian enzymes and play an essential role in host digestion. Interindividual variability of microbiota structure, and consequently of the expression of its genes (microbiome), was largely ascribed to the nutritional regime. Diet influences microbiota composition and function with short- and long-term effects. In spite of the vast literature, molecular mechanisms underlying these effects still remain elusive. In this review, we summarized the current evidence on the role exerted by gut microbiota and, more specifically, by its metabolites in the establishment of the host epigenome. The interest in this topic stems from the fact that, by modulating DNA methylation and histone modifications, the gut microbiota does affect the cell activities of the hosting organism.
For more details about article, please follow: https://doi.org/10.3390/nu12030597
Targeting Microbiota 2021 Congress
October 20-22, 2021 - Paris, France & Online
www.microbiota-site.com
FMT 2021: Reorganization and Perspective

Prof. Peter C. Konturek, President of ISM from Teaching Hospital of the University of Jena, Germany will present a talk entitled "FMT 2021: Reorganization and Perspective".
Prof. Konturek will present new data on FMT and include important remarks on the reorganization of fecal microbiota therapy due to the SARS Cov2 Pandemic.
Targeting Microbiota 2021 Congress
October 20-22, 2021 - Paris, France & Online
www.microbiota-site.com
The Microbiome of IBD and the Potential of Machine Learning
 Dr. Marcus Claesson from University College Cork, Ireland will join the Targeting Microbiota 2021 Virtual Congress scheduled on October 20-22, 2021, and will give a presentation entitled "The Microbiome of IBD and the Potential of Machine Learning".
Dr. Marcus Claesson from University College Cork, Ireland will join the Targeting Microbiota 2021 Virtual Congress scheduled on October 20-22, 2021, and will give a presentation entitled "The Microbiome of IBD and the Potential of Machine Learning".
The talk will cover two of the largest published microbiome studies of inflammatory bowel disease. In addition to highlighting how combinations of omics data can deepen host-microbiome insights, promising avenues for diagnostics and prognostics using Machine Learning will be discussed.
Targeting Microbiota 2021 Congress
October 20-22, 2021 - Paris, France & Online
www.microbiota-site.com
Human Gut Microbes and COVID-19
 Prof. Lanjuan Li from Zhejiang University, China will join the Targeting Microbiota 2021 which will be organized on October 20-22, 2021 and give a major presentation entitled "Human Gut Microbes and COVID-19".
Prof. Lanjuan Li from Zhejiang University, China will join the Targeting Microbiota 2021 which will be organized on October 20-22, 2021 and give a major presentation entitled "Human Gut Microbes and COVID-19".
Prof. Li will highlight these three points :
1. Alterations of gut microbiota in patients with COVID-19.
2. Prediction value of gut microbiota in diagnose and illness severity of COVID-19.
3. The potential value of probiotic intervention in the treatment of COVID-19.
Targeting Microbiota 2021 Congress
October 20-22, 2021 - Paris,France & Online
www.microbiota-site.com
An Ecological Framework to Understand the Efficacy of Fecal Microbiota Transplantation
 Prof. Yang-Yu Liu from Harvard Medical School & Brigham and Women's Hospital, USA will join the Targeting Microbiota 2021 Congress which will be held on October 20-22, 2021 and give a presentation entitled "An Ecological Framework to Understand the Efficacy of Fecal Microbiota Transplantation".
Prof. Yang-Yu Liu from Harvard Medical School & Brigham and Women's Hospital, USA will join the Targeting Microbiota 2021 Congress which will be held on October 20-22, 2021 and give a presentation entitled "An Ecological Framework to Understand the Efficacy of Fecal Microbiota Transplantation".
Summary of the talk: Here we present an ecological framework to understand the efficacy of fecal microbiota transplantation (FMT) in treating conditions associated with a disrupted gut microbiota, using the recurrent Clostridioides difficile infection as a prototype disease. This framework predicts several key factors that determine the efficacy of FMT. Moreover, it offers an efficient algorithm for the rational design of personalized probiotic cocktails to decolonize pathogens. We analyze data from both preclinical mouse experiments and a clinical trial of FMT to validate our theoretical framework. The presented results significantly improve our understanding of the ecological principles of FMT and have a positive translational impact on the rational design of general microbiota-based therapeutics.
Targeting Microbiota 2021 Congress
October 20-22, 2021 - Paris,France & Online
www.microbiota-site.com
Mitochondrial dysfunction caused by bacteria activates intrinsic apoptosis and inflammation
Monash Biomedicine Discovery Institute (BDI) scientists have discovered a previously unknown method used by bacteria to evade immune responses.
The study, published in Nature Microbiology, points to potential new ways of countering bacterial infections, which are becoming increasingly resistant to antibiotics.First author Dr Pankaj Deo said researchers in Dr Thomas Naderer’s laboratory took a different approach to understanding the process by which bacteria release toxins that disarm the “powerhouse” mitochondria in immune cells.The study showed that immune cells sense that their mitochondria are no longer functional during infections, which triggers apoptosis. “Ironically, it is the activation of host cell death factors that deliver the final blow to mitochondria which induces apoptosis, not the bacterial toxins themselves,” Dr Pankaj said.The researchers genetically targeted apoptotic factors and showed that they were able to reduce inflammation in mice, which increased health outcomes.They used the bacterial pathogens Neisseria gonorrhoeae, uropathogenic Escherichia coli and the deadly Pseudomonas aeruginosa, prevalent in hospitals and which can be multi-drug resistant. However, the findings would apply to other species of bacteria too, Dr Deo said.Dr Naderer, who oversaw the research, said that understanding the ways some bacterial infections evade immune response by targeting mitochondria opens new therapeutic possibilities.“There’s been a lot of effort trying to block endotoxins that kill immune cells but this study really shifts the focus onto different toxins that might be more important,” Dr Naderer said.“It gives us a few good leads that we can look at as a next step,” he said.“We’ve shown in this paper that we can accelerate the immune response,” he said. “The other side is that if that response persists and we get constant inflammation – which is usually associated with bacterial infection and which causes a lot of tissue damage – we have a new way to shut down that tissue-damaging inflammation.”“What scientists have thought before is that when endotoxins are released by bacteria they induce an inflammatory type of programmed cell death called pyroptosis in immune cells,” Dr Deo said. Endotoxins are part of the external cell wall of essentially all Gram-negative bacteria.“We’ve found that the pathogenic bacteria use a similar mechanism to release additional toxins,” he said. “They kill immune cells by releasing small surface structures called outer membrane vesicles – packages of toxins that target mitochondria. The mitochondria are disarmed, become dysfunctional then die according to apoptosis or cellular suicide.”The scientists will investigate drugs that are now advancing to the clinic, and at repurposing drugs already in use, perhaps as anti-cancer treatments, to see if they can be used to clear bacterial infections.
News source: www.technologynetworks.com
Article: Deo et al. (2020). Mitochondrial dysfunction caused by outer membrane vesicles from Gram-negative bacteria activates intrinsic apoptosis and inflammation. Nature Microbiology. DOI: https://doi.org/10.1038/s41564-020-0773-2
First successful delivery of mitochondria to liver cells in animals
University of Connecticut researcher Dr. George Wu recently published a paper in the Journal of Gastroenterology and Hepatology outlining his successful experiment delivering mitochondria to liver cells.
This groundbreaking experiment marks the first time researchers have ever successfully introduced mitochondria into specific cells in living animals.
Mitochondria generate energy from the conversion of fatty acids and carbohydrates to carbon dioxide and water, powering cells throughout the body. There is a significant link between mitochondrial damage and various liver diseases. When mitochondria are damaged, they cannot provide the liver with enough energy to function normally. This results in liver cell death and liver failure.
Currently, the only treatment for liver failure is a complete organ transplant. Surgeons perform approximately 8,000 liver transplants per year in the United States, but because of a shortage of donor livers, thousands more people on the waitlist for a transplant will die before receiving one.
Using their knowledge of a well-characterized receptor on the liver, Wu and his team previously showed mitochondria can be coated with certain carrier proteins which lead the liver to recognize them and take them up. These proteins have exposed galactose, a kind of sugar, on their surface. The galactose acts as a signal for the liver to internalize that protein.
“We took advantage of a normal, natural mechanism,” Wu says.
This paper finds that healthy mitochondrial complexes can be delivered to the livers of living rats through simple intravenous injection.
The team harvested mitochondria from mouse specimens. The mitochondria were mixed with a protein carrier and purified to form complexes which could be taken into the liver.
Along with the mitochondria, Wu injected a peptide which facilitated the release of the mitochondria once they reached the cells. This peptide allowed the mitochondria to be taken up into the liver cells’ cytoplasm rather than digested, which is what the liver does to most molecules it internalizes.
“If you don’t have that, mitochondria might be targeted to liver cells, but they would be destroyed,” Wu says.
At the end of the experiment, Wu and his colleagues found approximately 27% of the total injected mitochondria were detected in the liver, a significant proportion for therapeutic use.
Less than 2% were found in the spleen and less than 1% in the lungs, suggesting the uptake was not random and evenly distributed throughout all organs. In other words, the researchers were successful in creating a protein coating that made mitochondria specifically identifiable and internalized by the liver.
The achievement was far from a foregone conclusion, since mitochondria do not normally travel through the blood stream. The many potentially lethal obstacles made it a harrowing journey to the liver by way of veins to the heart, the lungs, and finally through arteries to the liver and the rest of the body. The coated mitochondria were apparently able to survive contact with blood cells, blood proteins, narrow blood vessels, and potential attacks from the immune system.
“To me, it’s rather amazing that we could detect any donor mitochondria at all,” Wu says. “When you consider all the obstacles that could get in the way.”
While this experiment only measured the short-term effects of mitochondrial transplant, there are potential long-term benefits.
Mitochondria have their own DNA and RNA, meaning they can reproduce independently of the rest of the cell. The transplanted mitochondria may replicate with the cells during cell division.
All cells have a certain number of mitochondria they need to sustain their activities. Wu speculates that cells may gradually eliminate the damaged mitochondria as the healthy, donor mitochondria increase in number until they have the proper number of healthy mitochondria.
The next step for this research is to test the method with rats who have mitochondrial liver damage. This will display clinical relevance for potentially developing this technique for treatment of liver diseases.
This process has the potential to address a serious gap in treatment for liver diseases. It may even eventually be used to treat other maladies throughout the body affected by mitochondrial malfunction or damage.
Wu is now working with UConn’s tech transfer group and has been issued a patent on targeted transplantation of mitochondria to hepatocytes. The University has filed patent protection on the proof of principle in animals. For more information about this technology for partnering and/or licensing contact Ana Fidantsef ([email protected]).
Wu received an M.D. and Ph.D. from the Albert Einstein College of Medicine in biochemistry and medicine. He completed his residency at the Harlem Hospital Center, Columbia College of Physicians and Surgeons, and fellowship training in gastroenterology-hepatology at Albert Einstein College of Medicine. He is currently a professor of medicine, Chief of Hepatology, and holds the Herman Lopata Chair in Hepatitis Research. His clinical interests include general gastroenterology, endoscopy, ultrasound-assisted liver biopsy and ultrasound-assisted paracentesis, but the focus is on liver disease.
News Source: www.uconn.edu
Influenza: combating bacterial superinfection with the help of the microbiota
 Technology vector created by brgfx - www.freepik.com
Technology vector created by brgfx - www.freepik.com
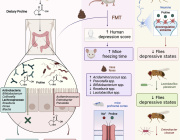
Researchers from the Lille Centre for Infection and Immunity (CNRS/INSERM/Institut Pasteur de Lille/University of Lille/CHU Lille), INRAE and from Brazilian (Belo Horizonte), Scottish (Glasgow) and Danish (Copenhagen) laboratories have shown for the first time in mice that perturbation of the gut microbiota caused by the influenza virus favours secondary bacterial superinfection. Published in Cell Reports on March 3, 2020, these results open up new prospects for the prevention and treatment of bacterial pneumonia, a major cause of death in elderly or vulnerable people infected with the influenza virus.
Influenza and its complications continue to be a significant public health concern as well as a major social and economic burden. Vaccination campaigns, together with the discovery of new antiviral therapies, provide preventive and therapeutic solutions. However, impairment of defence mechanisms against secondary bacterial infections, which considerably worsen the clinical picture of people with influenza, remains a major problem.
Specializing in the field of pulmonary immunity, a team led by François Trottein, a CNRS researcher at the Lille Centre for Infection and Immunity focused on the gut microbiota, well known for their key role in many physiological processes, including immune defence mechanisms. Scientists have shown that, in mice, influenza temporarily alters the composition and metabolic activity of the gut microbiota, probably due to reduced food consumption during illness. During influenza, the production of short-chain fatty acids by the bacteria of the microbiota is also diminished. The team has now shown that these fatty acids remotely favour the bactericidal activity of macrophages present in the lungs. Perturbation of the intestinal microbiota by influenza thus compromises lung defences, particularly against Streptococcus pneumoniae, the leading cause of bacterial pneumonia in humans.
The researchers also showed that this sensitivity to bacterial superinfection can be corrected by treatment with acetate, one of the main short-chain fatty acids produced by the microbiota. Their work could have practical applications for the well-being of infected patients, who would be better protected against influenza-related complications. This work was made in collaboration with scientists from the Micalis Institute (INRAE/AgroParistech/Université Saclay), the Lille Inflammation Research International Center (INSERM/Université de Lille/CHU Lille), the Laboratory of Design and Application of Bioactive Molecules (CNRS/University of Strasbourg), the Molecular Virology and Immunology Unit (INRAE) and GenoScreen (Lille), the Universidade Federal de Minas Gerais (Belo Horizonte, Brazil), the Institute of Molecular, Cell and Systems Biology (Glasgow, Scotland) and the Department of Pharmacology (University of Copenhagen, Denmark). This discovery represents a major breakthrough in the understanding of the mechanisms behind bacterial superinfections in influenza patients. It could lead to the development of new nutritional and/or therapeutic strategies to better control bacterial infections.
News source: https://www.eurekalert.org/
Analysis of oral microbiome from fossil human remains revealed the significant differences in virulence factors of modern and ancient Tannerella forsythia
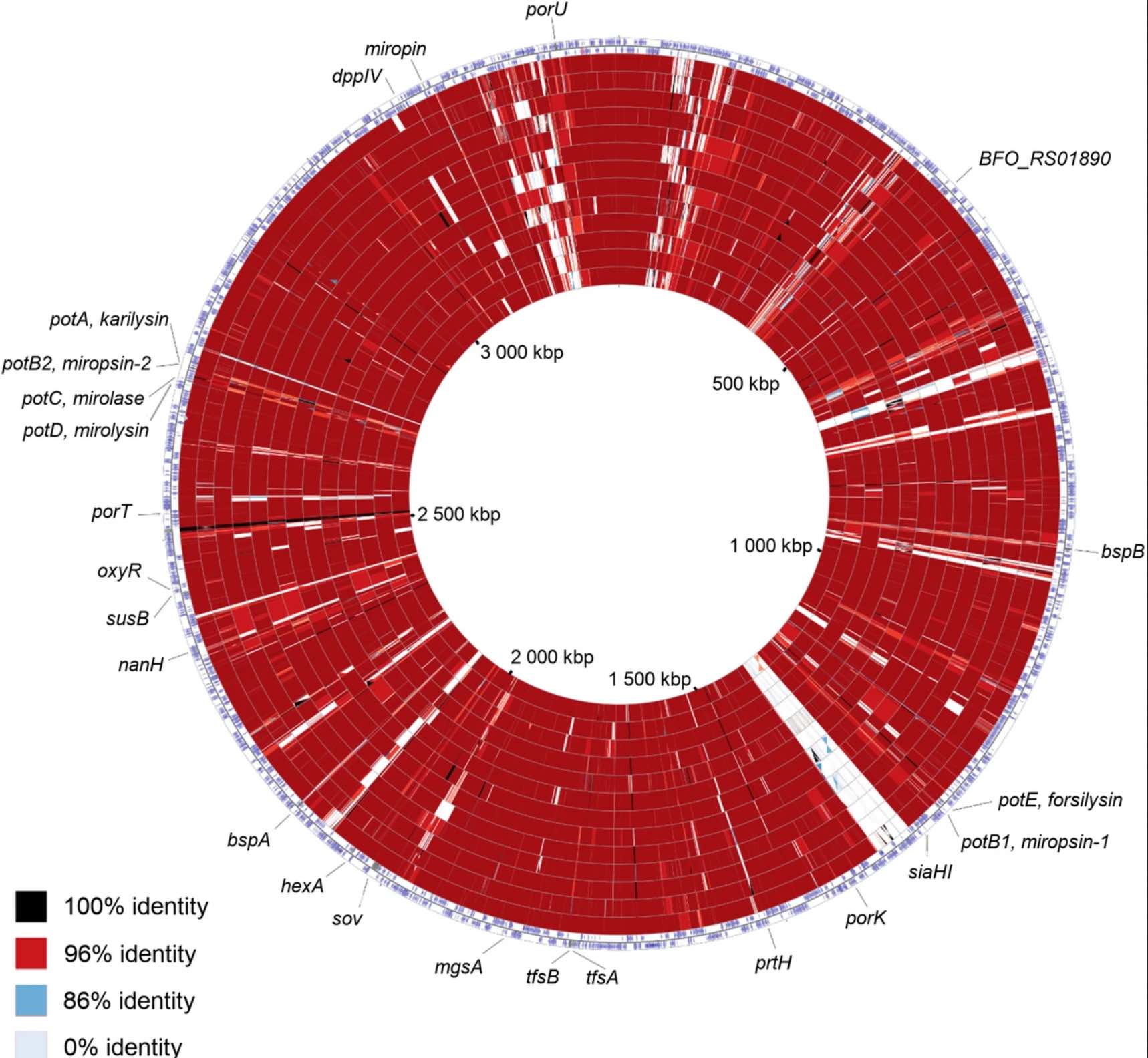 Figure: ©biomedcentral.com
Figure: ©biomedcentral.comBackground
Recent advances in the next-generation sequencing (NGS) allowed the metagenomic analyses of DNA from many different environments and sources, including thousands of years old skeletal remains. It has been shown that most of the DNA extracted from ancient samples is microbial. There are several reports demonstrating that the considerable fraction of extracted DNA belonged to the bacteria accompanying the studied individuals before their death.
Results
In this study we scanned 344 microbiomes from 1000- and 2000- year-old human teeth. The datasets originated from our previous studies on human ancient DNA (aDNA) and on microbial DNA accompanying human remains. We previously noticed that in many samples infection-related species have been identified, among them Tannerella forsythia, one of the most prevalent oral human pathogens. Samples containing sufficient amount of T. forsythia aDNA for a complete genome assembly were selected for thorough analyses. We confirmed that the T. forsythia-containing samples have higher amounts of the periodontitis-associated species than the control samples. Despites, other pathogens-derived aDNA was found in the tested samples it was too fragmented and damaged to allow any reasonable reconstruction of these bacteria genomes. The anthropological examination of ancient skulls from which the T. forsythia-containing samples were obtained revealed the pathogenic alveolar bone loss in tooth areas characteristic for advanced periodontitis. Finally, we analyzed the genetic material of ancient T. forsythia strains. As a result, we assembled four ancient T. forsythia genomes - one 2000- and three 1000- year-old. Their comparison with contemporary T. forsythia genomes revealed a lower genetic diversity within the four ancient strains than within contemporary strains. We also investigated the genes of T. forsythia virulence factors and found that several of them (KLIKK protease and bspA genes) differ significantly between ancient and modern bacteria.
Conclusions
In summary, we showed that NGS screening of the ancient human microbiome is a valid approach for the identification of disease-associated microbes. Following this protocol, we provided a new set of information on the emergence, evolution and virulence factors of T. forsythia, the member of the oral dysbiotic microbiome.
News source: www.bmcgenomics.biomedcentral.com
Article Reference: Philips, A., Stolarek, I., Handschuh, L. et al. Analysis of oral microbiome from fossil human remains revealed the significant differences in virulence factors of modern and ancient Tannerella forsythia. BMC Genomics 21, 402 (2020). https://doi.org/10.1186/s12864-020-06810-9
Personalized whole‐body models integrate metabolism, physiology, and the gut microbiome
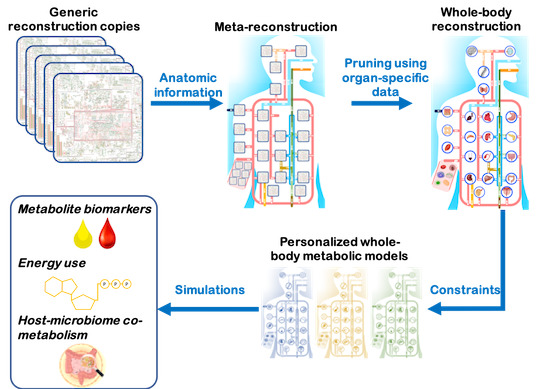 Image from: Mol Syst Biol (2020)16:e8982
Image from: Mol Syst Biol (2020)16:e8982
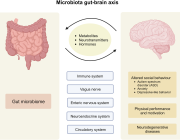
Comprehensive molecular‐level models of human metabolism have been generated on a cellular level. However, models of whole‐body metabolism have not been established as they require new methodological approaches to integrate molecular and physiological data. We developed a new metabolic network reconstruction approach that used organ‐specific information from literature and omics data to generate two sex‐specific whole‐body metabolic (WBM ) reconstructions. These reconstructions capture the metabolism of 26 organs and six blood cell types. Each WBM reconstruction represents whole‐body organ‐resolved metabolism with over 80,000 biochemical reactions in an anatomically and physiologically consistent manner. We parameterized the WBM reconstructions with physiological, dietary, and metabolomic data. The resulting WBM models could recapitulate known inter‐organ metabolic cycles and energy use. We also illustrate that the WBM models can predict known biomarkers of inherited metabolic diseases in different biofluids. Predictions of basal metabolic rates, by WBM models personalized with physiological data, outperformed current phenomenological models. Finally, integrating microbiome data allowed the exploration of host–microbiome co‐metabolism. Overall, the WBM reconstructions, and their derived computational models, represent an important step toward virtual physiological humans.
News Source: www.embopress.org
Journal Reference:
Personalized whole‐body models integrate metabolism, physiology, and the gut microbiome
Ines Thiele, Swagatika Sahoo, Almut Heinken, Johannes Hertel, Laurent Heirendt, Maike K Aurich, Ronan MT Fleming
Mol Syst Biol. (2020) 16: e8982 https://doi.org/10.15252/msb.20198982
At your Agenda:
8th World Congress on Targeting Microbiota
October 22-23, 2020 - UNESCO, Paris, France
www.microbiota-site.com
A New Blood Component Revealed
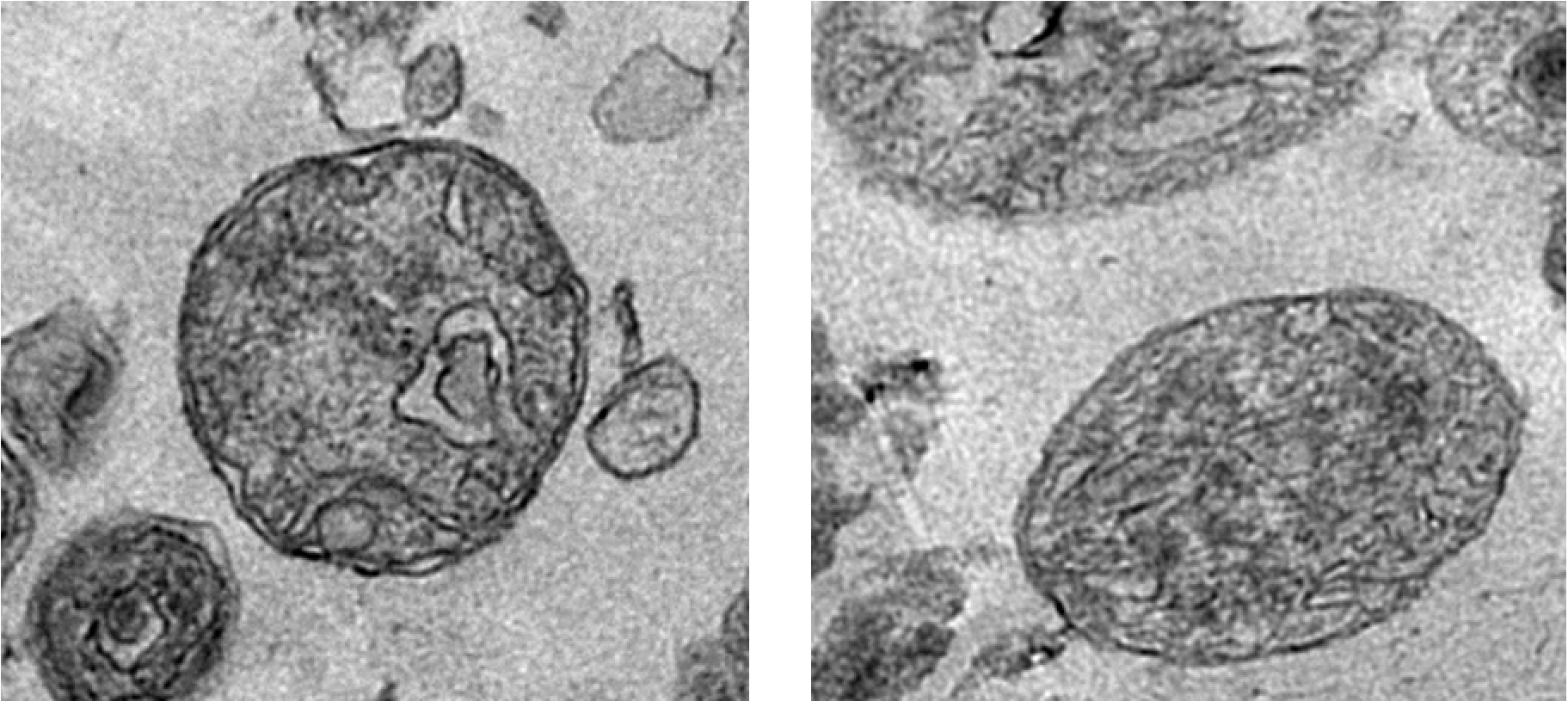
Functional extracellular mitochondria revealed in the blood circulation. ©Alain R. Thierry/Inserm
Does the blood we thought to know so well contain elements that had been undetectable until now? The answer is yes, according to a team of researchers from Inserm, Université de Montpellier and the Montpellier Cancer Institute (ICM) working at the Montpellier Cancer Research Institute (IRCM), which has revealed the presence of whole functional mitochondria in the blood circulation. These organelles that are responsible for cellular respiration had hitherto only been found outside cells in very specific cases. The team’s findings, published in The FASEB Journal, will deepen our knowledge of physiology and open up new avenues for treatment.
Mitochondria are organelles that are found in the eukaryotic cells. A place of cellular respiration, they are the cells’ “batteries” and play a major role in energy metabolism and intercellular communication. Their particularity is to possess their own genome, transmitted solely by the mother and separate from the DNA contained in the nucleus. The mitochondria can sometimes be observed outside the cells in the form of fragments encapsulated within microvesicles. Under certain very specific conditions the platelets are also capable of releasing intact mitochondria into the extracellular space.
The work of a team led by Inserm researcher Alain R. Thierry at the Montpellier Cancer Research Institute (Inserm/Université de Montpellier/Montpellier Cancer Institute) has now revolutionized knowledge of this organelle by revealing that whole functioning extracellular mitochondria are in fact found in the bloodstream!
The researchers used previous findings which showed that the plasma of a healthy individual contains up to 50,000 times more mitochondrial DNA than nuclear DNA. They hypothesized that for it to be detectable and quantifiable in the blood in this manner, the mitochondrial DNA had to be protected by a structure of sufficient stability. In order to identify such a structure, plasma samples from around 100 individuals were analyzed.
This analysis revealed the presence in the blood circulation of highly stable structures containing whole mitochondrial genomes. Following examination of their size and density, as well as the integrity of their mitochondrial DNA, these structures observed using electron microscopy (up to 3.7 million per ml of plasma) were revealed to be intact and functional mitochondria.
Throughout the seven-year research period, the scientists used as many technical and methodological approaches as possible to validate this presence of circulating extracellular mitochondria in the blood.
“When we consider the sheer number of extracellular mitochondria found in the blood, we have to ask why such a discovery had not been made before, notes Thierry. Our team has built up expertise in the specific and sensitive detection of DNA in the blood, by working on the fragmentation of extracellular DNA derived from the mitochondria in particular”, he adds.
But what is the role of these extracellular mitochondria? The answer to that could be linked to the structure of the mitochondrial DNA, similar to that of bacterial DNA, which gives it the ability to induce immune and inflammatory responses. Based on this observation, the researchers hypothesize that these circulating mitochondria could be implicated in many physiological and/or pathological processes requiring communication between the cells (such as the mechanisms of inflammation). Indeed, recent studies have demonstrated the ability of certain cells to transfer mitochondria between themselves, such as the stem cells with damaged cells. “The extracellular mitochondria could perform various tasks as messenger for the entire body”, specifies Thierry.
In addition to its importance to our knowledge of physiology, this discovery could lead to improvements in the diagnosis, monitoring and treatment of certain diseases. In fact, the research team is now devoting its attention to evaluating the extracellular mitochondria as biomarkers in non-invasive prenatal diagnosis and cancer.
Inserm press room A New Blood Component Revealed
Link : https://presse.inserm.fr/en/a-new-blood-component-revealed/37905/
Phage infection mediates inhibition of bystander bacteria
Bacteriophages (phages) are being considered as alternative therapeutics for the treatment of multidrug resistant bacterial infections. Considering phages have narrow host-ranges, it is generally accepted that therapeutic phages will have a marginal impact on non-target bacteria. We have discovered that lytic phage infection induces transcription of type VIIb secretion system (T7SS) genes in the pathobiont Enterococcus faecalis. Membrane damage during phage infection induces T7SS gene expression resulting in cell contact dependent antagonism of diverse Gram positive bystander bacteria. Deletion of essB, a T7SS structural component abrogates phage-mediated killing of bystanders. A predicted immunity gene confers protection against T7SS mediated inhibition and disruption of an upstream LXG toxin gene rescues growth of E. faecalis and Staphylococcus aureus bystanders. Phage induction of T7SS gene expression and bystander inhibition requires IreK, a serine/threonine kinase, and OG1RF_11099, a predicted transcriptional activator. Our findings highlight how phage infection of a target bacterium can affect non-target bystander bacteria and implies phage therapy could impose collateral damage to polymicrobial communities.
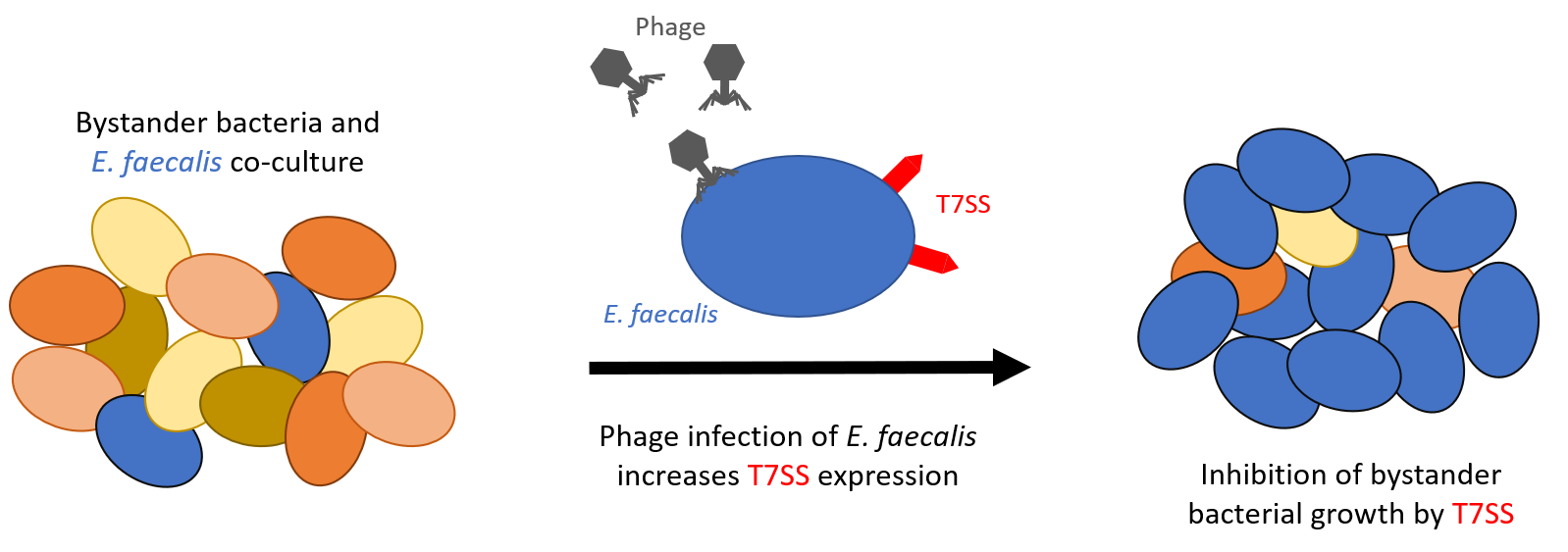
Image source: prelights.biologists.com
News Source: www.biorxiv.org
At your Agenda:
8th World Congress on Targeting Microbiota
October 22-23, 2020 - UNESCO, Paris, France
www.microbiota-site.com
Lung Microbiota Predict Clinical Outcomes in Critically Ill Patients
Rationale: Recent studies have revealed that in critically ill patients, lung microbiota are altered and correlate with alveolar inflammation. The clinical significance of altered lung bacteria in critical illness is unknown. Objectives: To determine if clinical outcomes of critically ill patients are predicted by features of the lung microbiome at the time of admission. Methods: We performed a prospective observational cohort study in an intensive care unit (ICU) at a university hospital. Lung microbiota were quantified and characterized using droplet digital PCR and bacterial 16S rRNA gene sequencing. Primary predictors were the bacterial burden, community diversity, and community composition of lung microbiota. The primary outcome was ventilator-free days, determined at 28 days post admission. Measurements and Main Results: Lungs of 91 critically ill patients were sampled using miniature-bronchoalveolar lavage within 24 hours of ICU admission. Patients with increased bacterial lung bacterial burden had fewer ventilator-free days (HR 0.43, CI 0.21-0.88), which remained significant when controlled for pneumonia and severity of illness. The community composition of lung bacteria predicted ventilator-free days (P=0.003), driven by the presence of gut-associated bacteria (e.g. Lachnospiraceae and Enterobacteriaceae spp.). Detection of gut-associated bacteria was also associated with the presence of the acute respiratory distress syndrome. Conclusions: Key features of the lung microbiome (bacterial burden, enrichment with gut-associated bacteria) predict outcomes in critically ill patients. The lung microbiome is an understudied source of clinical variation in critical illness, and represents a novel therapeutic target for the prevention and treatment of acute respiratory failure.
Authors: Robert P. Dickson , Marcus J. Schultz , Tom van der Poll , Laura R. Schouten , Nicole R. Falkowski , Jenna E Luth , Michael W. Sjoding , Christopher A Brown , Rishi Chanderraj , Gary B. Huffnagle , Lieuwe D.J. Bos
Patients with ankylosing spondylitis display distinct fecal microbiota signature
 Patients with ankylosing spondylitis demonstrate a distinct fecal microbiota signature that is linked to levels of fecal calprotectin, but not other clinical parameters, according to data published in Arthritis Research & Therapy.
Patients with ankylosing spondylitis demonstrate a distinct fecal microbiota signature that is linked to levels of fecal calprotectin, but not other clinical parameters, according to data published in Arthritis Research & Therapy.
“The gastrointestinal tract is the home of more than 1,000 species of bacteria, but also fungi and viruses, which coexist with the host in a reciprocal relationship,” Eva Klingberg, MD,PhD, of the Sahlgrenska Academy at the University of Gothenburg, in Sweden, and colleagues wrote. “The gut microbiota is necessary for the development and shaping of the immune system, and the host genetics play a role in the establishing and shaping of the gut microbiota.”
“Intestinal microbiota most likely play a role in initiating and triggering the immune system in individuals who are genetically susceptible for [inflammatory bowel disease], leading to the typical gut inflammation of [Crohn’s disease] and [ulcerative colitis],” they added. “Aberrations in the gut microbiome, dysbiosis with decreased bacterial diversity, expansion of potentially pro-inflammatory bacteria, and reduction of potentially anti-inflammatory, protective bacteria have repeatedly been shown in IBD. However, it is still unclear whether the dysbiosis in IBD is a cause or a consequence of the gut inflammation.”
To evaluate differences in fecal microbiota among patients with AS, ulcerative colitis and healthy individuals, as well as analyze the relationship between fecal microbiota, fecal calprotectin and disease-related variables in AS, Klingberg and colleagues studied a cohort from Western Sweden across the 5-year period. Participants included 204 patients recruited in 2009, with the same patients invited back for a follow-up in 2014. For this analysis, the researchers focused on 150 participants with AS, 18 with ulcerative colitis and 17 healthy controls with no history of gastrointestinal or other chronic disorders.
Klingberg E, et al. Arthritis Res Ther. 2019;doi:10.1186/s13075-019-2018-4.
News source: https://www.healio.com/
The Poster Contribution Award was discerned to Dr. Agata Chudzik
 The Poster Contribution Award was discerned to Dr. Agata Chudzik from Medical University of Lublin, Poland, during Targeting Microbiota 2019, which was held in Krakow, Poland, for her poster presentation entitled "Evaluation of neurometabolites level after microbiotic supplementation (Lactobacillus rhumnosus LR-JB1TM) in rat model of chronic unpredictable mild stress".
The Poster Contribution Award was discerned to Dr. Agata Chudzik from Medical University of Lublin, Poland, during Targeting Microbiota 2019, which was held in Krakow, Poland, for her poster presentation entitled "Evaluation of neurometabolites level after microbiotic supplementation (Lactobacillus rhumnosus LR-JB1TM) in rat model of chronic unpredictable mild stress".
The study assesses the neurometabolic and behavioral changes after bacterial diet in an animal model of chronic unpredictable mild stress. The results show that JB-1 bacteria diet influenced the stabilization of stress-related neurometabolites in rat brain and prevented to develop anxiety/depressive-like behavior.
Testimonial by Dr. Chudzik:
"It was a great pleasure to attend to Targeting Microbiota 2019 Congress. I would like to thank Organizers for the opportunity to present my PhD research and I feel very honoured that Scientific Committee awarded it.Participating in Targeting Microbiota Congress allowed me to hear and discuss the recent advances and challenges in the microbiota research. The outstanding lectures given by experts in the field of human and animal microbiome were very inspiring and enabled a new look at the topics discussed. I have enriched my knowledge about the microbiome-gut-brain axis in detail, which is a particularly exciting issue for me. Moreover congress allowed me to meet people interested in similar issues and every conversation led to brainstorming and sharing ideas."
Media center:
International Society of Microbiota
Contact: [email protected]
www.microbiota-site.com
The Short Oral Contribution Award was delivered to Dr. Satu Pekkala
 During the Targeting Microbiota 2019 congress, which was held in Krakow, Poland, the Scientific Committee awarded Dr. Satu Pekkala, from University of Jyvaskyla, Finland, for her Oral Presentation entitled "Targeting Faecalibacterium prausnitzii with prebiotic xylo oligosaccharides prevents from Non-Alcoholic Fatty Liver Disease (NAFLD)"
During the Targeting Microbiota 2019 congress, which was held in Krakow, Poland, the Scientific Committee awarded Dr. Satu Pekkala, from University of Jyvaskyla, Finland, for her Oral Presentation entitled "Targeting Faecalibacterium prausnitzii with prebiotic xylo oligosaccharides prevents from Non-Alcoholic Fatty Liver Disease (NAFLD)"
The study presented by Dr. Pekkala identifies F. prausnitzii as target treat NAFLD and the underlying preventive mechanisms that involve fat oxidation and glucose metabolism.
Testimonial by Dr. Pekkala:
" I am very honored for this award and I congratulate the International of Society of Microbiota by organizing the congress 7th Targeting Microbiota full of excellent presentations. I have always thought that the gut microbiota studies should advance from detecting associations to show mechanistically the biological significance of the detected associations. This is needed to be able to target the microbes to treat different diseases in the future. Therefore, I am thrilled to see the development of the gut microbiota studies from year to year in this congress!
I am currently Academy of Finland Research Fellow at the Faculty of Sport and Health Sciences, University of Jyväskylä, Finland. I am also Adjunct Professor at the Faculty of Medicine, University of Turku, Finland. My research team has discovered that a specific gut bacterium, Faecalibacterium prausnitzii associated with liver fat content in humans. We treated a murine model of fatty liver with this bacterium and found that it was able to prevent the disease. Unfortunately, the potential therapeutic microbes are not always accepted for human use and therefore it is important to find alternative ways to increase their natural abundance in the gut. As I presented in the congress, we found a prebiotic fibre that increased F. prausnitzii in vivo and at the same could prevent fatty liver in rats. We are currently setting up a human study to see whether our findings can be translated to humans."
Media center:
International Society of Microbiota
Contact: [email protected]
www.microbiota-site.com
Prof. Chantal Pichon, French National Centre for Scientific Research, France, awarded by International Society of Microbiota
International Society of Microbiota (ISM) is honored to announce the winner of the Scientific Contribution Award for the year 2019, Prof. Chantal Pichon from French National Centre for Scientific Research, University of Orléans, France.

Prof. Chantal Pichon gave an excellent communication during the 7th World Congress on Targeting Microbiota 2019, which was held in Krakow, Poland, on the Dialogue between the skin and the microbiota: The immune system is under control.
Testimonial of Prof. Pichon:
"I am indebted to the organizers for inviting me to speak at Targeting Microbiota and giving me the award. It was an inspiring and very high level conference. Cutting Edge research projects have been presented indicating how fast is growing the knowledge in the field and how we could together tackle the challenges on developing novel therapies based on microbiota interaction with our body."
Oral presentation of Prof. Chantal Pichon during Targeting Microbiota 2019:
The skin, the largest organ of our body is also the outermost barrier of the organism. It protects from external harm and senses danger signals. It contains a sophisticated immune system that involves armada of immune cells comprising keratinocytes, resident antigen-presenting cell, innate lymphoid cells, innate-like cells, and adaptive tissue-resident memory cells and molecular mediators. The skin hosts also microbial communities recognized as skin microbiota that constitutes one living response barrier to environmental factors. Those microorganisms live in complete harmony with the immune players and are involved in the epithelial barrier reinforcement. Under homeostasis, an established alliance of immunity and microbiota intermingles innate and adaptive branches of the immune system. Under various types of stress, the symbiotic relationship changes into a dysbiotic one resulting in skin disorders. The talk will be focused on the cutaneous immune system from cellular to molecular actors and how skin microbiota can acts to limit pathogenic microbial invasion and to promote various aspects of immune responses.



For media information:
International Society of Microbiota
Media center:
Contact: [email protected]
www.microbiota-site.com
Development and Investment Landscape in the Microbiome
 During the congress, Dr. Peter Bak, Back Bay Life Science Advisors, USA will provide his vision and recommendations.
During the congress, Dr. Peter Bak, Back Bay Life Science Advisors, USA will provide his vision and recommendations.
The title of his presentation is 'Corporate Development in the Microbe Space: Has Investment Outpaced Science?'
1) Microbiome Company Landscape
2) Current State of Clinical Development
3) Investment and Corporate Considerations
Prof. Marvin Edeas and his team presented a study on Intelligence Artificial and Health meeting at University Paris-Sud
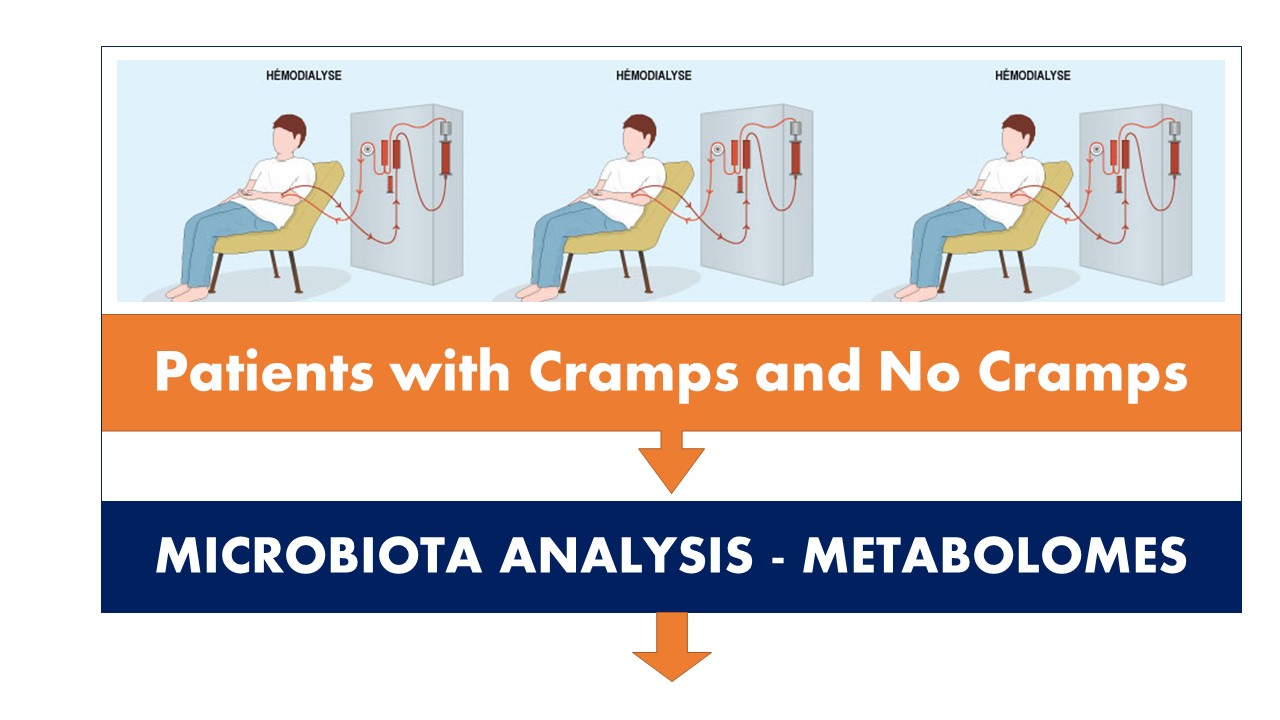
The Intelligence Artificial and Health meeting was held on November 25, 2019, at University Paris-Sud. During the meeting, Prof. Marvin Edeas from INSERM - U1016, Institut Cochin, Hôpital Cochin, University Paris Descartes with his team presented a study entitled "Artificial Intelligence in the Healthcare Industry: The Hemodialysis patients with cramps initiative".
Introduction
Artificial intelligence in the healthcare industry will open a huge opportunity. Demand for basic and advanced analytics is growing exponentially in healthcare. We are beginning to enter into an era where life sciences and data science are merging. Our initiative will collect clinical data from hemodialysis patients, which will urgently need a Data Scientists to process it. To be able to analyze and make recommendations based on this data, we need AI.
Methods
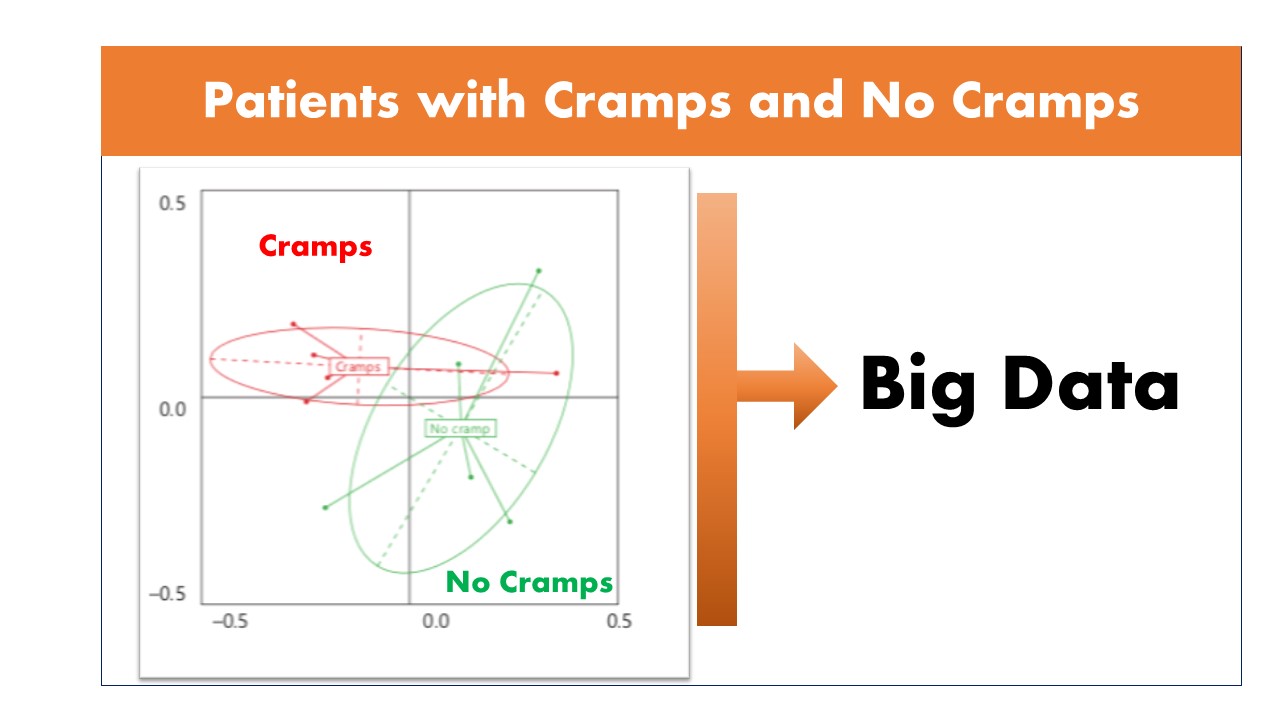
For the first time we published in 2018 a possible link between Microbiota quality, mitochondrial activity and the occurrence of muscle cramps in hemodialysis patients using citrate dialysate. Our objectives were to assess the gut microbiota quality, mitochondrial activity, and to investigate their possible relationship with HD-associated muscle cramp (HDAMC). This clinical study revealed a possible dysbiosis of microbiota and mitochondrial dysfunction in HD patients with cramps under CD compared to patients without cramp.
Confirmation of our observations is required in a large-scale study, which could demonstrate a novel pathophysiological mechanism linking muscle cramps to gut microbiota HD patients. We now have a cohort of more than 1500 patients to complete our study.
Results Expected
We will use a combined microbiome and metabolome signatures in feces and metabolome in plasma as new biomarkers to identify in upstream the HD patients with altered metabolism in order to propose an adapted therapy. Advances in sequencing technologies could provide opportunities to understand not only the taxonomy but also the functional diversity of gut microbiota. These investigations could allow to justify the use of antibiotics, probiotics, prebiotics, or fecal microbiota transplantation to modulate gut microbiota as curatives or preventive strategies of muscle cramps. We will also look for a predictive marker of muscle cramps by initiating an Artificial Intelligence program.
Conclusion
There are significant hurdles to be overcome with our hemodialysis initiative: 1-finding talented data scientists. 2- regulatory aspects. This presentation will address these considerations.
Durand, P.-Y., Nicco, C., Serteyn, D., Attaf, D. & Edeas, M. Microbiota Quality and Mitochondrial Activity Link with Occurrence of Muscle Cramps in Hemodialysis Patients using Citrate Dialysate: A Pilot Study.
Blood Purif. 46, 301–308 (2018).
For more info, please check the agenda here: http://www.medecine.u-psud.fr/fr/recherche/colloque-intelligence-artificielle-et-sante.html

Prof. Peter Kuntorek and Prof. Marvin Edeas from the International Society of Microbiota will talk during the 9th Latvian Gastroenterology Congress
 Prof. Peter Konturek from Teaching Hospital of the University of Jena, Germany will talk about "Fecal microbiota transplantation as a powerful tool for modulating of gut".
Prof. Peter Konturek from Teaching Hospital of the University of Jena, Germany will talk about "Fecal microbiota transplantation as a powerful tool for modulating of gut".More Articles...
- Final Agenda & Abstracts Book of Microbiota 2019 Congress
- The International Society of Microbiota is pleased to welcome Biocrates Life Sciences AG to the 7th Targeting Microbiota World Congress 2019 !
- DNA Genotek received the ISM Industrial Award during Targeting Microbiota 2019
- Microbiota and Autism: Recent Advances and Perspectives